TAMS/TNT
TAMS/TNT: Novel Sagertech Communications diagnsotic and treatment solution for neuropathy induced fecal incontenence.
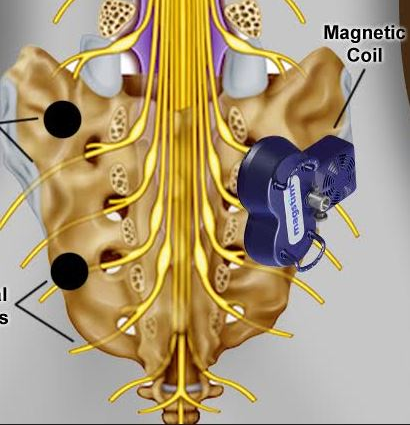
Translumbosacral Anorectal Magnetic Stimulation
Anorectal disorders disrupt the lives of 15-20% of people globally. This FDA-approved, CPT-code accessible, non-invasive, and highly effective diagnostic and therapy solution for patients with neuropathy-related fecal incontinence has proven over 75% efficacy in clinical studies. Sagertech Communications aims to position TAMS/TNT to close the gap between diagnosis and treatment and help patients manage their condition.
Translumbosacral Anorectal Magnetic Stimulation (TAMS) revolutionizes diagnosis of anorectal disorders. TAMS has applications for anal and rectal neuropathy among patients exhibiting fecal incontinence, urinary incontinence, pelvic floor disorders and spinal cord injury.
Translumbosacral Neuromodulation Therapy (TNT), a cutting edge non-invasive treatment leveraging magnetic technology for treatment of fecal incontinence, anorectal pain and pelvic floor disorders.
*TAMS test and TNT is reimbursed by most major insurances, including Medicare.
What Is TAMS?
Pioneered over 10 years of research by Dr. Satish Rao, a leading gut motility physician expert, TAMS is a physiological test with an innovative designed to comprehensively evaluate anorectal and pelvic floor neurophysiology using magnetic resonance
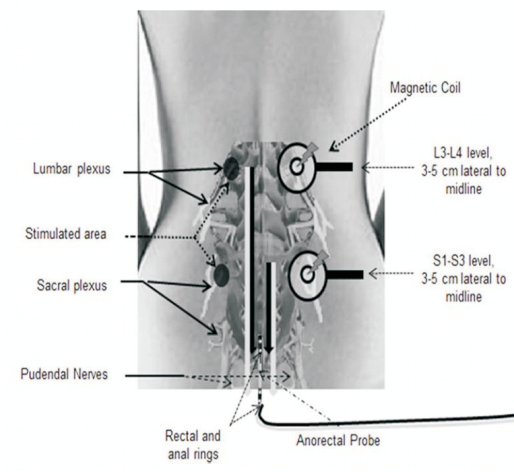
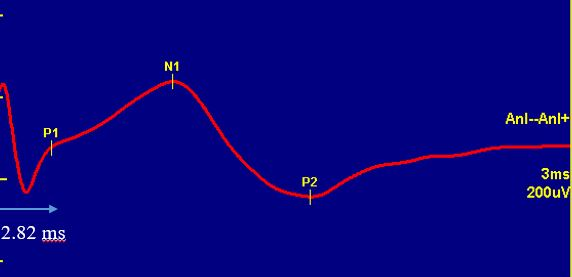
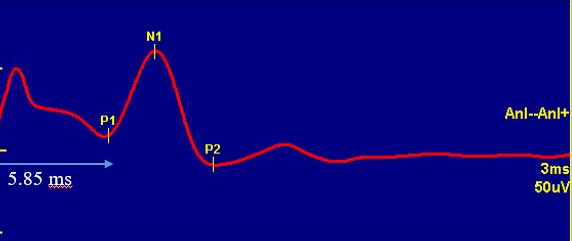
What Does TAMS measure ?
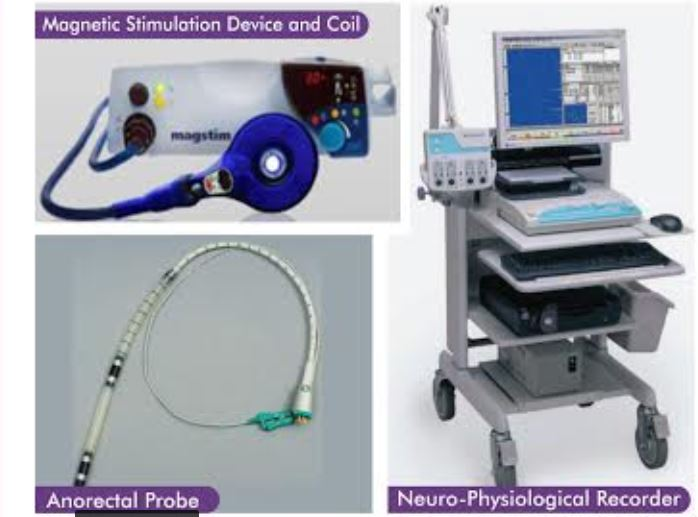
TAMS Test Equipment
Our equipment package includes a 3 part platform:
- Magnetic Stimulation Device
- Anorectal probe w/ 4 ring electrodes for detecting the MEP from the rectum and anal regions.
- Neuro-physiological recorder for recording the MEP reads.
Sagertech Communications has exclusive partnership agreements to provide our platform equipment package at an affordable price, globally.
TNT (Translumbosacral Neuromodulation Therapy)
- Perform TAMS test to measure MEP in FI patients
- Perform TNT sessions of Repetitive magnetic stimulation at each of the four identified Lumbo-sacral regions
- 600 simulations per site; Total of 2400 stimulations using the rapid magnetic stimulation device
- TAMS test is conducted at the end of therapy to evaluate neuropathy.
- Typically require six sessions once a week
- Reimbursed thru CPT codes by most medical insurances including Medicare
TAMS/TNT Test is Backed By 10 YEARS OF RESEARCH
TAMS has been rigorously tested in several controlled trials. It has been shown to be superior for detecting anorectal neuropathy in patients with fecal incontinence and in spinal cord injury.
TAMS test was an independent predictor of physiological dysfunction in fecal incontinence.
Recent study confirmed that TAMS detects neuropathy in over 85% of patients with FI.
TAMS is being used to evaluate fecal incontinence in a NIH-FIT trial at 4 Tier-1 Research Institutions at American academic universities.
Recent studies confirmed TNT significantly improves afferent ano-cortical signaling, efferent lumbo-anal and sacro-anal neuropathy and anorectal sensorimotor function.
